
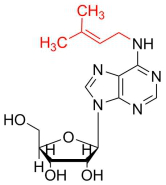
| Comman Name | N6-isopentenyladenosine |
| Symbol | i6A |
| Chemical Formula | C15H21N5O4 |
| Nucleoside Mass | 335.36 |
| Modification | Organisms or Cells | Contents | References |
| i6A | mouse liver | 1.20±0.55% [i6A/G] | https://pubmed.ncbi.nlm.nih.gov/24261999/ |
| i6A | mouse liver | 0.012±0.008% [i6A/G] | https://pubmed.ncbi.nlm.nih.gov/24261999/ |
| i6A | E. coli W3110 | 1.81% [i6A/Ψ] | https://pubmed.ncbi.nlm.nih.gov/6755395/ |
| i6A | E. coli K12 Hfr3000 | 1.45% [i6A/Ψ] | https://pubmed.ncbi.nlm.nih.gov/6755395/ |
| i6A | E. coli C-1 | 0.48% [i6A/Ψ] | https://pubmed.ncbi.nlm.nih.gov/6755395/ |
| i6A | E. coli B | 0.21% [i6A/Ψ] | https://pubmed.ncbi.nlm.nih.gov/6755395/ |
| i6A | E. coli 15 | 0.60% [i6A/Ψ] | https://pubmed.ncbi.nlm.nih.gov/6755395/ |
| i6A | E. coli W | 0.94% [i6A/Ψ] | https://pubmed.ncbi.nlm.nih.gov/6755395/ |
| i6A | S. typhimurium LT2 | 1.45% [i6A/Ψ] | https://pubmed.ncbi.nlm.nih.gov/6755395/ |
| i6A | S. typhimurium LT7 | 1.02% [i6A/Ψ] | https://pubmed.ncbi.nlm.nih.gov/6755395/ |
| i6A | LT2 AA2202 | 1.02% [i6A/Ψ] | https://pubmed.ncbi.nlm.nih.gov/6755395/ |
| i6A | A. th. cell culture | 5.4 [i6A/1000 tRNA molecules] | https://pubmed.ncbi.nlm.nih.gov/23417961/ |
| i6A | A. th. plants | 27.6 [i6A/1000 tRNA molecules] | https://pubmed.ncbi.nlm.nih.gov/23417961/ |




